Author:
Laura McKinney
Date Of Creation:
8 August 2021
Update Date:
1 May 2024

Content
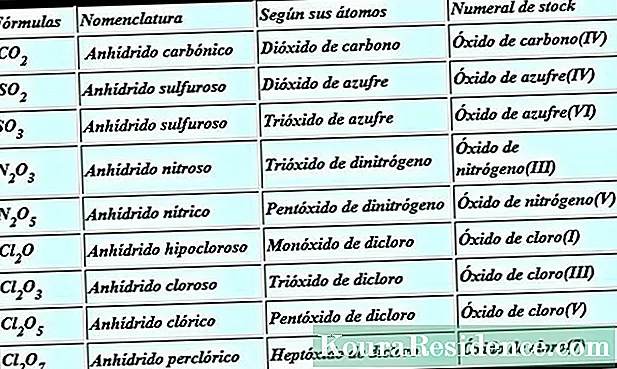
Thehydrides They are chemical compounds that combine hydrogen atoms (whose oxidation state is, in most cases, -1) and atoms of any other element on the periodic table in their molecule.
Three categories of hydrides are recognized:
- Metallic metallic: They are those that are formed with the alkaline and alkaline-earth elements, that is, with those that are further to the left of the periodic table of elements. They are non-volatile compounds that exhibit conductivity. Hydrogen is found in them as the hydride ion H¯. Within this group one can distinguish the hydrides that form the most electropositive metals (from groups 1 and 2); these hydrides are often called saline. Saline hydrides are generally white or gray solids that are obtained by direct reaction of metal with hydrogen at high temperatures.
- Volatile or non-metallic hydrides:They are those that are formed with non-metallic elements but little electronegative, specifically, with nitrogen, phosphorus, arsenic, antimony, bismuth, boron, carbon and silicon: all these receive specific names, beyond the general nomenclature; they are all metalloids or metals from the p block. They can also be called molecular or covalent hydrides, because they have covalent bonds. They form minerals of quite particular aspects. Silane, a hydride in this group, is of growing interest for its value for the manufacture of nanoparticles.
- Hydrogen hydrides:(also called simply hydracids) correspond to the combination of hydrogen with a halogen (fluorine, chlorine, bromine or iodine) or with an antigenic element (oxygen, sulfur, selenium, tellurium); only in the latter case does hydrogen act with its positive oxidation number (+1) and the other element is the one that works with a negative oxidation number (-1 in halogens, -2 in amphogens).
Examples of hydrides
- Sodium hydride (NaH)
- Phosphine (PH3)
- Barium hydride (BaH2)
- Bismutin (Bi2S3)
- Permanganic hydride (MnH7)
- Ammonia (NH3)
- Arsine (AsH3)
- Stibinite or antimonite
- Hydrobromic acid (HBr)
- Borano (BH3)
- Methane (CH4)
- Silane (SiH₄)
- Hydrofluoric acid (HF)
- Hydrochloric acid (HCl)
- Ferrous hydride (FeH3)
- Hydroiodic acid (HI)
- Hydrogen sulfide (H2S)
- Selenhydric acid (H2Se)
- Telluridic acid (H2Te)
- Lithium hydride (LiH)
Uses of hydrides
The uses of hydrides include those of desiccants and reducers, some are used as pure hydrogen sources.
Calcium hydride is especially useful as organic solvent drying agent. Sodium hydride requires great care in handling, as it reacts violently with water and can ignite.
If a fire occurs due to the ignition of this hydride, do not use water to extinguish it, because it would produce more flames. These fires are put out with powder fire extinguishers.