The temperature measurement units represent the physical magnitude of heat level of a body, or an environment. Temperature is a property associated with the movement of particles that exist in bodies and in the air, and based on it, they are determined different properties of bodies, of which probably the most notorious is the state: it is common to see this in water, where the temperature determines if the same body (water) will be in solid, liquid or gaseous state.
The same occurs with all substances, being able to determine in each of them the temperature point from which it will be solid below and above liquid (melting point) and the temperature point of which will be liquid at the bottom and gaseous above (evaporation point).
Therefore, the physical property of temperature is essential for the treatment of bodies and matter, and with this it is essential to be able to quantify it. Throughout history they were appearing different ways of measuring temperature, functional for different cases. The three most important ones will be detailed, in chronological order of their appearance:
- The degree Fahrenheit it was proposed in 1724, and it was determined under three points in such a way that its account does not pursue a dynamic of direct proportionality. Its use is widespread in the United States, for non-scientific uses.
- The degree Celsius was introduced in 1742, and the determination of its magnitude was made under the idea of degrees of freezing and boiling of water0 Celsius being the point at which water turns from a solid (ice) to a liquid (or vice versa), and 100 Celsius the level at which, once exceeded, the water boils and transforms into steam. This scale is used for most everyday temperatures in most parts of the world, but it is also commonly found in different types of scientific studies.
- Finally, the Kelvin degree was contributed in the mid-19th century, and is known as a absolute temperature level since it places its 0 point at the lowest energy level, that is, the point at which the particles lack motion. In this sense, there is no 0 Kelvin, and at a potential point of this type all substances would become solid. It is common for scientific use and practically null for everyday use, and it is not symbolized with the degree sign (°) because it is not a gradual but an absolute magnitude.
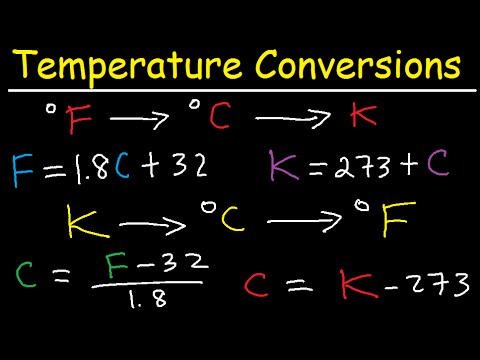
In this order of things, the three different temperatures must have clear mechanisms to be converted. Here are the six possible transformations between temperature units, and how they should be done correctly
- From Celsius to Kelvin: KELVIN = CELSIUS + 273.15
- From Celsius to Fahrenheit: FARENHEIT = (CELSIUS) * 9/5 + 32
- From Fahrenheit to Celsius: CELSIUS = (FARENHEIT - 32) * (5/9)
- From Fahrenheit to Kelvin: KELVIN = (FARENHEIT - 32) * (5/9) + 273.15
- Kelvin to Celsius: CELSIUS = KELVIN - 273.15
- From Kelvin to Fahrenheit: FARENHEIT = ((KELVIN - 273.15) * 9/5) + 32
From the operations seen, some examples of conversions can be mentioned to make it clearer.
- 300 K = 26.85 ° C
- 80 ° C = 176 ° F
- 25 ° C = 298.15 K
- 125 K = -148.15 ° C
- 250 ° C = 176 ° F
- 250 K = -9.67 ° F
- 100 ° C = 373.15 K
- 80 K = -315.67 ° F
- 800 K = 526.85 ° C
- 300 K = 80.33 ° F
- 20 ° C = 68 ° F
- 5 ° C = 41 ° F
- 30 ° F = -1.11 ° C
- 100 ° F = 37.77 ° C
- 15 ° F = 263,706K


